- AdventHealth
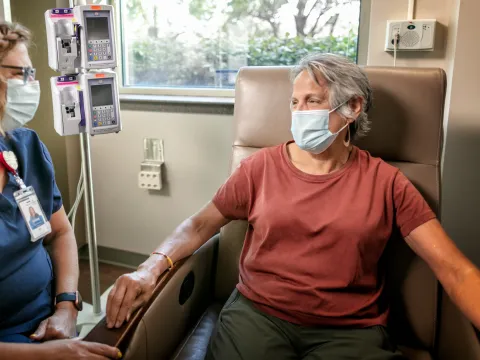
Those at high risk for severe COVID-19 now have access to a potentially lifesaving antibody treatment that can reduce the likelihood of becoming seriously ill from COVID-19. This treatment, offered at AdventHealth’s locations across the country, is the latest in a suite of COVID-19 therapeutics aimed at helping those with COVID-19 recover safely.
A single dose of monoclonal antibodies bamlanivimab and etesevimab administered together has been found to lower the chance of hospitalization or ER visits for people with mild-to-moderate COVID-19 who are at greater risk of hospitalization due to their age or a chronic medical condition, according to the FDA.*
Monoclonal antibody combinations, which are secured by the federal government and then distributed to states, contain proteins that mimic the immune system’s ability to fight the virus. The monoclonal antibody combinations that are FDA emergency use authorized include bamlanivimab and etesevimab, as well as casirivimab and imdevimab, commonly known as Regeneron.*
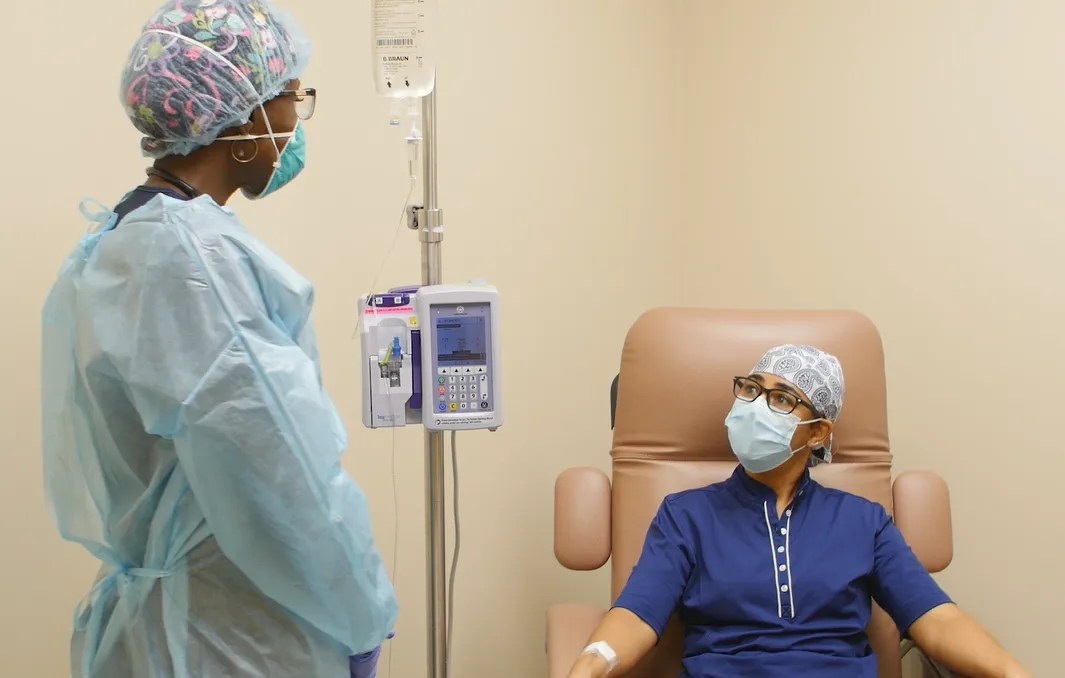
Doctors are able to prescribe the treatment to patients with an onset of symptoms within the past 10 days who have high-risk factors such as diabetes, heart disease or obesity and are not hospitalized or on oxygen. Some AdventHealth locations have opened dedicated antibody treatment clinics where patients can comfortably receive the hour-long intravenous infusion.
“We are thrilled to offer this lifesaving treatment to those at high risk for severe COVID-19 as a way to reduce hospitalizations and allow safe recoveries from the comfort of their own home,” said Brent Box, MD, chief medical officer for AdventHealth.
Monoclonal antibody treatments join a host of other COVID-19 therapies offered at AdventHealth including remdesivir, sarilumab, dexamethasone and convalescent plasma. The health system hopes to continue increasing its capacity for administering this treatment as federal supply distribution allows.
*This article was updated based on new information from the FDA, released on April 16, 2021.
Recent News

Local leaders, officials, and construction workers gathered today to commemorate a major milestone in the expansion underway at AdventHealth Daytona Beach: placing the final steel beam.

Dr. Jeffrey Keen, a board-certified orthopedic surgeon specializing in adult reconstruction, orthopedic surgery, robotic-assisted surgery, and sports medicine, has returned to AdventHealth Medical...

In recognition of National Donate Life Month, nearly 300 transplant patients and their families enjoyed AdventHealth’s 2025 transplant reunion.

According to the National Kidney Foundation, more than 101,000 people are currently on the organ transplant list in need of a new kidney. However, only about 17,000 transplants happen each year —...

The AdventHealth Board of Directors has appointed David Banks as the organization’s new president/CEO, effective immediately.

In life, Sophie Davis touched dozens of hearts. In passing, one of her organs could possibly save thousands of lives.

As the days get longer and the weather warms up, people are eager to get back to their favorite outdoor spring activities and sports. This transition from winter to spring often leads to an increased...

Marie Williams remembers being admitted to AdventHealth Parker on September 1, 2023, for colon resection surgery, but after that, things get hazy.

AdventHealth for Children is a nationally recognized children’s hospital and comprehensive care network caring for nearly 200,000 children annually.

Care Management Assistant, Meghan Bussard, was the recipient of the 2024 Outstanding Team Member Award for AdventHealth Shawnee Mission. Bussard is known across AdventHealth Shawnee Mission as a...

As the only physician in Central Florida certified to use the intestinal ultrasound, a cutting-edge diagnostic tool still new to the U.S., Dr. Jennifer Seminerio-Diehl is transforming the way IBD is...

Every March, we celebrate Women’s History Month and the countless contributions women have made across all parts of society, including health care. One such area where women have made remarkable...
