- AdventHealth
After nearly 10 years as part of the TrialNet studies, physicians at AdventHealth Central Florida Division are now administering the world’s first treatment to delay the onset of Type 1 diabetes (T1D). While we always thought this disease was incurable, the treatment AdventHealth researchers helped contribute to is now approved by the U.S. Food and Drug Administration (FDA) and available to patients in Central Florida, giving hope that T1D will one day be completely curable.

“This is a significant breakthrough in the treatment of Type 1 diabetes,” shared Anna Casu, M.D., an investigator in T1D research at the AdventHealth Translational Research Institute and lives with Type 1 diabetes herself. “For the first time, a disease we thought was inevitable, can now be delayed.”
On Jan. 11, Konda Reddy, M.D., medical director of pediatric endocrinology and diabetes at AdventHealth for Children became the first in Central Florida and one of the first in the country to begin treating a patient with Tzield (teplizumab-mzwv). This is the first drug in the world to delay the onset of stage 3 T1D in adults and pediatric patients 8 years and older who currently have stage 2 T1D.

“Diabetes is a disease that never goes away, so delaying its onset can have a significant impact on quality of life for patients and their families,” said Dr. Reddy. “The approval of Tzield is a historic moment for the Type 1 diabetes community. It means there’s more time to live without the burden of the disease and its complications.”
An intravenous infusion delivered daily over the course of 14 days, Tzield is a CD3-directed monoclonal antibody treatment. It binds to CD3 antigens presented on the surface of T cells, delaying the onset of stage 3 T1D, when blood sugar levels are high, and symptoms typically begin.
Tzield’s safety and efficacy were evaluated in a randomized, double-blind, event-driven, placebo-controlled trial conducted by the Type 1 diabetes TrialNet (Trial Network) consortium. With over 200,000 participants screened globally since launch, AdventHealth served as one of the screening sites (U.S, Canada, and Europe) for TrialNet studies.
In the trial, 76 patients with stage 2 T1D randomly received Tzield or a placebo once daily via intravenous infusion for 14 days. Over a median follow-up period of 51 months, 45% of the 44 patients who received Tzield were later diagnosed with stage 3 T1D, compared to 72% of the 32 patients who received a placebo. The mid-range time from randomization to stage 3 T1D diagnosis was 50 months for the patients who received Tzield and 25 months for those who received a placebo.
According to the Juvenile Diabetes Research Foundation, approximately 1.45 million Americans are living with T1D, and 64,000 are diagnosed each year in the U.S. An autoimmune disease, T1D causes a person’s immune system to destroy insulin-producing cells, elevating blood glucose to dangerous levels. Those with T1D require daily insulin via injection or pump to survive.
Tzield is approved for people who are at high risk for T1D and have abnormal blood sugar levels determined by an oral glucose tolerance test. To meet the criteria for “high risk,” a person must have a screening test that confirms the presence of at least two specific autoantibodies that indicate the body is already attacking the insulin-producing beta cells in the pancreas.
To refer a pediatric patient for T1D screening or Tzield treatment, click here.
To learn more about diabetes research at the AdventHealth Translational Research Institute, click here.
Recent News
The AdventHealth Neuroscience Institute is the first in Florida and one of the first in the country to begin recruiting patients with primary progressive or non-active secondary progressive multiple...

Discover what’s being accomplished in Central Florida to bridge the health gap with Orange County Mayor Jerry Demings and AdventHealth’s Dr. Alric Simmonds.
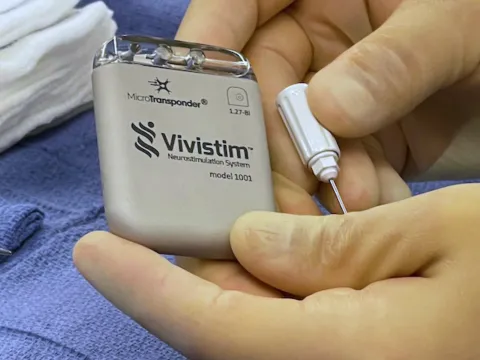
Breakthrough device offers new hope for stroke survivors struggling with rehabilitation following ischemic stroke
The Convergent Hybrid Ablation procedure has been gaining acceptance as an effective treatment option for long-standing persistent atrial fibrillation (AFib) since the CONVERGE trial data published in...

Recently, AdventHealth for Children pediatric orthopedic surgeon Sean Keyes, DO, Katelyn Smith, PA-C, and their team performed their 100th bridge-enhanced anterior cruciate ligament (ACL) repair (BEAR...
AdventHealth recently began piloting a new Genomics Risk Assessment for Cancer and Early Detection (GRACE) program that combines the use of digital mammography, artificial intelligence (AI) technology...
AdventHealth Clinical Research Unit (CRU) Executive Director and Medical Director of Genitourinary Oncology Guru Sonpavde, MD, co-authored an article on the AMBASSADOR Phase III clinical trial results...
AdventHealth Research Institute is currently seeking physicians experiencing exhaustion, irritability, hopelessness, anxiety, work/home imbalance or similar burnout symptoms to participate in a...

A promising new treatment for AFib patients called Pulse Field Ablation is first offered in Central Florida at AdventHealth and shows less damage to tissue.
Plastic and Reconstructive Microsurgeon Sabrina Pavri, MD, and Breast Surgeon Devina McCray, MD, recently began offering immediate neurotization after breast reconstruction, a new surgical technique...

Explore the healing power of music for stroke survivors and their families in this episode of the Inspiring Wholeness podcast.

Dr. Andrew Weinfeld will be responsible for executing clinical and organizational strategy, improving clinical effectiveness and patient safety, and delivering exceptional patient experiences for the...
