- AdventHealth
As part of Healthcare Innovation’s Advancing Digital Healthcare Delivery, a monthly digital health virtual series with innovators who are transforming healthcare, Rajan Wadhawan, M.D., neonatologist and senior executive officer of AdventHealth for Children and AdventHealth for Women, candidly discussed the clinical challenges of rare diseases and how a new board for the state of Florida is working to improve health outcomes for patients living with these diseases.
Healthcare Innovation Advancing Digital Healthcare Delivery with Rajan Wadhawan, M.D.
“Rare diseases are usually chronic and involve multiple systems, and almost all of these conditions have a genetic cause, which means they are manifesting in infants,” said Wadhawan. Because of these complexities, they are difficult to diagnose and manage, often causing a delay in care.
“Diagnosing a rare disease can take four to five years and oftentimes it includes an earlier misdiagnosis,” he said.
While rare diseases are classified as those that affect less than 200,000 people in the U.S., there are more than 7,000 different types of rare diseases – and each present with their own nuanced conditions and symptoms. All of these combined impacts an immense number of people: nearly 1 in 10 Americans are estimated to be living with rare diseases.
“The knowledge base for every condition is so vast, it is not possible for an average practicing physician to know everything about every rare disease,” Wadhawan said.

During his four-year term on Florida’s newly established Rare Disease Advisory Council (RDAC), Wadhawan will provide his expertise and personal perspective to encourage potential policy changes to make it easier for physicians and patients. This could include developing an online repository of searchable information to improve the diagnosis process and a consortium within the state or region to conduct rare disease research.
“Just like anything in life, there is so much that needs to be done that it can be overwhelming and daunting, but you have to start somewhere,” Wadhawan concludes. “We cannot be frozen by or paralyzed by the enormity of a challenge. If we first examine the areas where we can have the most impact, within the areas where we are experts, we can take it from there.”
As one of 20 representatives on Florida’s RDAC, Wadhawan and his colleagues are working to improve health outcomes for those with a rare disease and making recommendations to state leaders on critical issues, including the need for increased awareness, diagnostic tools and access to affordable treatments and cures.
Recent News
The AdventHealth Neuroscience Institute is the first in Florida and one of the first in the country to begin recruiting patients with primary progressive or non-active secondary progressive multiple...
Accurately determining food intake remains a challenge in nutrition research. A new study published in Nature Metabolism and co-authored by Dr. Corbin introduces a metagenomics-powered approach to...

Discover what’s being accomplished in Central Florida to bridge the health gap with Orange County Mayor Jerry Demings and AdventHealth’s Dr. Alric Simmonds.
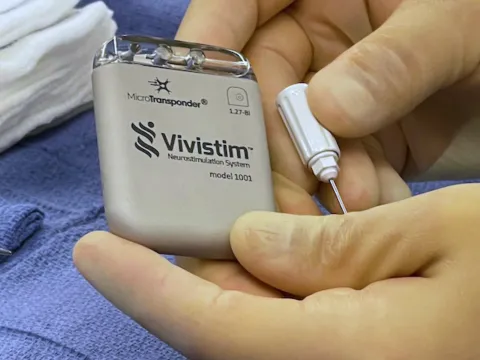
Breakthrough device offers new hope for stroke survivors struggling with rehabilitation following ischemic stroke
Jennifer Seminerio, MD, recently became one of the first in Florida to use intestinal ultrasound (IUS) to help assess and manage treatment of patients with inflammatory bowel disease (IBD). A non...
The Convergent Hybrid Ablation procedure has been gaining acceptance as an effective treatment option for long-standing persistent atrial fibrillation (AFib) since the CONVERGE trial data published in...

Recently, AdventHealth for Children pediatric orthopedic surgeon Sean Keyes, DO, Katelyn Smith, PA-C, and their team performed their 100th bridge-enhanced anterior cruciate ligament (ACL) repair (BEAR...
Physician leaders from AdventHealth’s emergency department, infectious disease, inpatient, pediatrics and pharmacy teams all collaborated to develop a respiratory virus testing algorithm to assist...
Thoracic surgeon Colleen Gaughan, MD, and her team at AdventHealth Celebration, recently became one of the first in the country to incorporate targeted imaging agent Cytalux (pafolacianine) as part of...

On the newest Inspiring Wholeness podcast, Obie Diaz, local morning radio show host, shares how a routine physical eventually led to two open heart surgeries.
AdventHealth recently began piloting a new Genomics Risk Assessment for Cancer and Early Detection (GRACE) program that combines the use of digital mammography, artificial intelligence (AI) technology...
AdventHealth Clinical Research Unit (CRU) Executive Director and Medical Director of Genitourinary Oncology Guru Sonpavde, MD, co-authored an article on the AMBASSADOR Phase III clinical trial results...
