- AdventHealth

Last fall, an AdventHealth physician and his team researched and developed a new brain-eating amoeba test which can produce results within three hours.
WHY IT MATTERS:
Previously, testing routinely took up to six days, while the amoeba kills its host in only 3-7 days, making identifying and treating the condition extremely urgent and difficult.
KEY UPDATE:
Over the past 10 months, Jose Alexander, M.D., clinical microbiologist and director of microbiology, virology and immunology for AdventHealth Orlando, and his team have run several experiments on their recently developed brain-eating amoeba test to further improve its results. The result is an extended viable testing window, which allows for simplified shipping and accelerates diagnosis, ultimately improving patient outcomes.
“We’ve run these Rapid Amoeba PCR tests through several sample transportation temperatures to determine sample stability and quality,” said Alexander. “Spinal fluid samples can now be shipped at room temperature if testing is expected within 48 hours and refrigerated if expected within seven days.”
Alexander believes the enhanced sample stability for rapid Amoeba PCR testing at different temperatures is a game changer because it enables faster, more effective treatment for patients by simplifying and expediting specimen packaging and shipment, still providing accurate and reliable results by direct testing on the sample upon arrival.
GO DEEPER:
The groundbreaking brain-eating amoeba test developed last fall has changed the landscape of how physicians treat this severe, and most of the time deadly, infection by cutting down the diagnosis time from days to hours. In fact, the brain-eating amoeba, also known as Naegleria fowleri, is one of several growing health concerns across the U.S. It kills more than 97% of people infected due to delays in identifying and treating the illness.
By going a step further and testing for stability and quality, Alexander and his team have developed a more efficient diagnostic tool for physicians allowing for a rapid turnaround time from collection to result, without delays due to sample freezing, special packaging and the ability to still provide reliable and clinically actionable results. This sample shipping is available for cases that are too far from Orlando, like Miami or out of state.
“AdventHealth is accepting samples for testing from any hospital across the U.S.,” said Alexander.
For physicians with medical staff privileges at AdventHealth, this new test is currently available in Epic as “Amoeba Screen, CSF.” For physicians at AdventHealth locations not yet on Epic, the test is available as a send out to AdventHealth Orlando as a reference laboratory. All other physicians can contact Jose Alexander, M.D. at jose.alexander@adventhealth.com for more information or for potential cases.
To read more about the recently developed test used to detect brain-eating amoebas, learn about these deadly infections and discover how the team repurposed a test used for other viruses to drive the innovation journey, click here.
Recent News
The AdventHealth Neuroscience Institute is the first in Florida and one of the first in the country to begin recruiting patients with primary progressive or non-active secondary progressive multiple...
Accurately determining food intake remains a challenge in nutrition research. A new study published in Nature Metabolism and co-authored by Dr. Corbin introduces a metagenomics-powered approach to...

Discover what’s being accomplished in Central Florida to bridge the health gap with Orange County Mayor Jerry Demings and AdventHealth’s Dr. Alric Simmonds.
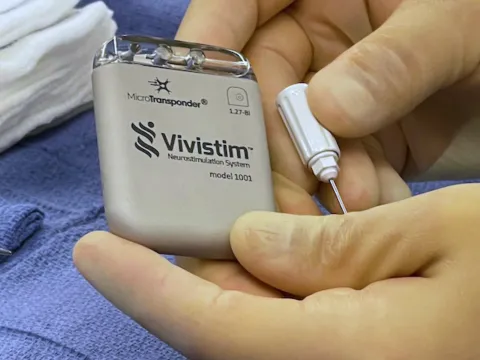
Breakthrough device offers new hope for stroke survivors struggling with rehabilitation following ischemic stroke
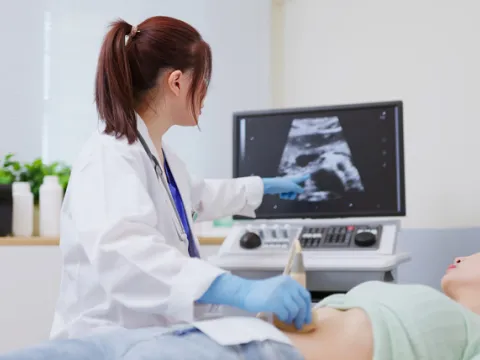
Jennifer Seminerio, MD, recently became one of the first in Florida to use intestinal ultrasound (IUS) to help assess and manage treatment of patients with inflammatory bowel disease (IBD). A non...
The Convergent Hybrid Ablation procedure has been gaining acceptance as an effective treatment option for long-standing persistent atrial fibrillation (AFib) since the CONVERGE trial data published in...

Recently, AdventHealth for Children pediatric orthopedic surgeon Sean Keyes, DO, Katelyn Smith, PA-C, and their team performed their 100th bridge-enhanced anterior cruciate ligament (ACL) repair (BEAR...
Physician leaders from AdventHealth’s emergency department, infectious disease, inpatient, pediatrics and pharmacy teams all collaborated to develop a respiratory virus testing algorithm to assist...
Thoracic surgeon Colleen Gaughan, MD, and her team at AdventHealth Celebration, recently became one of the first in the country to incorporate targeted imaging agent Cytalux (pafolacianine) as part of...

On the newest Inspiring Wholeness podcast, Obie Diaz, local morning radio show host, shares how a routine physical eventually led to two open heart surgeries.
AdventHealth recently began piloting a new Genomics Risk Assessment for Cancer and Early Detection (GRACE) program that combines the use of digital mammography, artificial intelligence (AI) technology...
AdventHealth Clinical Research Unit (CRU) Executive Director and Medical Director of Genitourinary Oncology Guru Sonpavde, MD, co-authored an article on the AMBASSADOR Phase III clinical trial results...
