- AdventHealth
On August 3, 2022, AdventHealth Celebration OB/GYN Erica Stockwell, DO, performed the first vaginal-approach hysterectomy in Central Florida using the Anovo™ Surgical System, the first and only FDA-authorized surgical robot designed to enable the minimally invasive transvaginal approach for benign gynecological procedures. Authorized uses include hysterectomy, salpingectomy, oophorectomy, adnexectomy, and ovarian cyst removal.
By incorporating a wrist, elbow and shoulder joint to mimic a surgeon’s movements, Momentis Surgical’s Anovo™ enables a transvaginal approach, providing patients with a quicker return to normal activities compared to abdominal hysterectomy.
“Anovo™ is a hybrid surgical approach that combines the technologic benefits of laparoscopy, like visualization and advanced energy, with the ergonomics of robotics and the optimal patient outcomes of vaginal surgery,” explains Dr. Stockwell.

Among U.S. women of reproductive age, hysterectomy is the second most frequently performed surgical procedure, with approximately 600,000 hysterectomies performed each year. In 2017, The American College of Obstetricians and Gynecologists (ACOG) released a statement that vaginal hysterectomy should be the approach of choice whenever feasible because evidence demonstrates that it is associated with better outcomes when compared with other approaches to hysterectomy. However, the less invasive vaginal approach has been used in only 16 percent of hysterectomies due to anatomical and technical limitations. These findings fueled the development of the Anovo™ system.
“We know vaginal hysterectomy provides the best patient outcomes, but poor visualization and access to the pelvis had inhibited its use,” shares Dr. Stockwell. “The Anovo™ system was specifically designed to overcome these barriers and make the vaginal approach more accessible. We believe this new technology will improve care for our patients who require surgery for benign gynecological conditions like abnormal uterine bleeding, fibroids, endometriosis, chronic pelvic pain, prolapse, pre-cancerous changes, and ovarian cysts. It will provide them with less pain and scarring, reduced infection rates, and a quicker recovery and return to their normal daily lives.”
AdventHealth has plans to be to be a primary site for the ongoing registry of clinical outcomes using the Anovo™ system with Dr. Stockwell serving as Primary Investigator.
If you would like to learn more about this procedure, click here.
Clinical Anovo Story
Recent News
The AdventHealth Neuroscience Institute is the first in Florida and one of the first in the country to begin recruiting patients with primary progressive or non-active secondary progressive multiple...
Accurately determining food intake remains a challenge in nutrition research. A new study published in Nature Metabolism and co-authored by Dr. Corbin introduces a metagenomics-powered approach to...
Discover what’s being accomplished in Central Florida to bridge the health gap with Orange County Mayor Jerry Demings and AdventHealth’s Dr. Alric Simmonds.
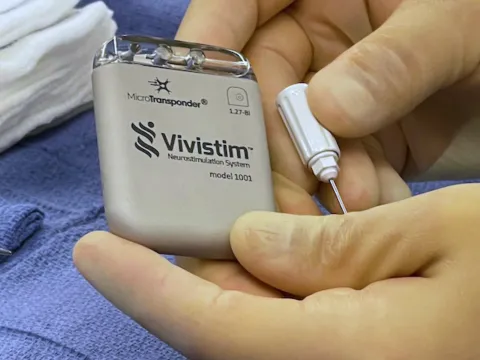
Breakthrough device offers new hope for stroke survivors struggling with rehabilitation following ischemic stroke
Jennifer Seminerio, MD, recently became one of the first in Florida to use intestinal ultrasound (IUS) to help assess and manage treatment of patients with inflammatory bowel disease (IBD). A non...
The Convergent Hybrid Ablation procedure has been gaining acceptance as an effective treatment option for long-standing persistent atrial fibrillation (AFib) since the CONVERGE trial data published in...
Recently, AdventHealth for Children pediatric orthopedic surgeon Sean Keyes, DO, Katelyn Smith, PA-C, and their team performed their 100th bridge-enhanced anterior cruciate ligament (ACL) repair (BEAR...
Physician leaders from AdventHealth’s emergency department, infectious disease, inpatient, pediatrics and pharmacy teams all collaborated to develop a respiratory virus testing algorithm to assist...
Thoracic surgeon Colleen Gaughan, MD, and her team at AdventHealth Celebration, recently became one of the first in the country to incorporate targeted imaging agent Cytalux (pafolacianine) as part of...
On the newest Inspiring Wholeness podcast, Obie Diaz, local morning radio show host, shares how a routine physical eventually led to two open heart surgeries.
AdventHealth recently began piloting a new Genomics Risk Assessment for Cancer and Early Detection (GRACE) program that combines the use of digital mammography, artificial intelligence (AI) technology...
AdventHealth Clinical Research Unit (CRU) Executive Director and Medical Director of Genitourinary Oncology Guru Sonpavde, MD, co-authored an article on the AMBASSADOR Phase III clinical trial results...