Treatment Available In Tampa Area
Advanced Care and Life-Changing Results
Atrial fibrillation (AFib) affects roughly 3 million Americans, putting them at increased risk for blood clots and stroke. However, medical advancements like the WATCHMAN and WATCHMAN FLX™ can help reduce that risk without open-heart surgery or using blood-thinning medications for life.
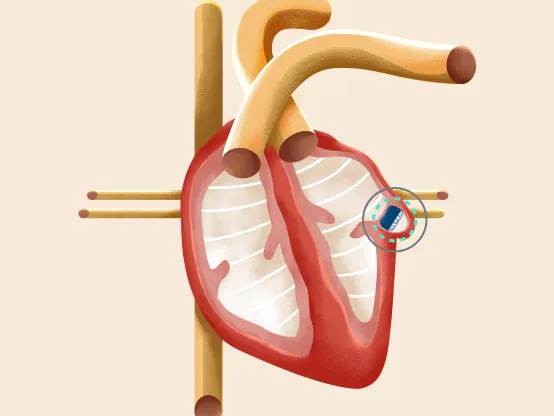
In patients with non-valvular AFib, more than 90% of blood clots originate from the heart. The WATCHMAN device permanently closes the left atrial appendage, preventing blood clots from entering the bloodstream, thus reducing stroke risk. Over time, this typically ends the need for blood thinners.
Answers to Common Questions About AFib and the WATCHMAN Device
-
Q:Question: Who is a candidate for the WATCHMAN FLX?
A:Answer:With the WATCHMAN FLX implant, one minimally invasive procedure reduces your stroke risk and can alleviate worries for life. If three or more of the following apply to you, contact our team at Call844-235-6555 to learn more.
Continue ReadingCollapse Answer- You have an atrial fibrillation diagnosis
- You have atrial fibrillation (AFib) not caused by a heart valve problem or you have been prescribed a blood thinner for AFib (aspirin is not a blood thinner)
- You have atrial fibrillation (AFib) not caused by a heart valve problem or you have been prescribed a blood thinner for AFib (aspirin is not a blood thinner)
- You have three or more of the following conditions:
- Abnormal kidney (renal) function
- Abnormal liver function
- Cancer
- Congestive heart failure
- Dementia or Alzheimer’s
- Diabetes
- Hypertension (elevated blood pressure)
- Stroke, mini-stroke or thromboembolism
- Vascular disease
- You’ve experienced the following side effects of blood thinners:
- Major bleeding (GI bleeding in the brain or head)
- Minor bleeding (nose bleeds or easy bruising)
- Bleeding risk from other health conditions
- Decreased stability or mobility
- Avoiding hobbies or work that puts you at risk of falling
- Missing activities because of fear of falling
- You have issues with blood thinners, including:
- Adverse side effects
- Difficulty remembering to take your blood thinners
- Difficulty staying within clotting range
- Difficulty keeping regular INR blood testing appointments
- Difficulty sticking to less than eight alcoholic drinks per week
- Prolonged use of aspirin or NSAIDs
- Other major issues
- You have an atrial fibrillation diagnosis
-
Q:Question: What happens during the WATCHMAN or WATCHMAN FLX procedure?
A:Answer:Implanting a WATCHMAN or WATCHMAN FLX is usually done using general anesthesia. You’ll have a breathing tube inserted, which will be removed before you are transferred to the recovery area.
Continue ReadingCollapse AnswerTo begin, your surgeon makes a small cut in your upper leg and inserts a flexible tube called a catheter into a vein in the left side of your heart. The WATCHMAN or WATCHMAN FLX moves through the catheter and is inserted into your left atrial appendage, forming a tight seal as heart tissue grows over the implant and permanently seals that area.
Once the procedure is complete, you’ll have several follow-up tests, including a physical exam, lab work and possibly an echocardiogram (heart ultrasound).
Since the procedure doesn’t require open-heart surgery, most people experience less pain and a faster recovery with fewer complications.
-
Q:Question: What happens after the WATCHMAN surgery?
A:Answer:Most patients stay in the hospital for one day. It’s common to have a sore throat from the breathing tube and the transesophageal echocardiogram performed during the procedure. You may be sore or have bruising in one or both areas of the groin, and you might have to remain on your back for up to eight hours so your providers can closely monitor your groin for bleeding or swelling.
Continue ReadingCollapse AnswerAfter discharge, you’ll continue taking blood thinners until your heart tissue grows enough to permanently seal your left atrial appendage, keeping it isolated from the rest of your heart. You can usually stop taking blood thinners within 45 days of your procedure. If you’re on Coumadin, you’ll need to have your INR checked within the week and be closely monitored every week to check the thinness of your blood.
Follow-up care may include a special diet, exercise and medication. Your physician will monitor your condition with regular appointments for at least two years to ensure the device continues to be effective.
-
Q:Question: Are these devices safe and effective?
A:Answer:The WATCHMAN and WATCHMAN FLX are the only implants of their kind that have earned approval from the Food and Drug Administration (FDA). They have been tested extensively and have a proven record of safe, effective treatment. More than 150,000 people worldwide have received a WATCHMAN or WATCHMAN FLX implant.
Continue ReadingCollapse AnswerThe risks are relatively low when a skilled cardiologist performs the procedure. In rare cases, however, the procedure could increase the risk of blood clots, infection and stroke.
The WATCHMAN device offers comparable results to those achieved with warfarin, including:
- 85% reduction in hemorrhagic stroke
- 63% reduction in disabling stroke
- 56% reduction in cardiovascular death
- 71% relative reduction in major bleeding after six months
- 99% of patients no longer require warfarin within the first year of their procedure
-
Q:Question: How do I know if I have AFib?
A:Answer:AFib occurs in the heart’s left atrium, which squeezes blood through the heart's chambers. When the upper chambers receive abnormal electrical signals, the heart suffers and begins to beat too fast and irregularly.
Continue ReadingCollapse AnswerFor most adults, a normal resting heart rate ranges between 60 and 100 beats per minute. For people with AFib, the resting heartbeat could go up to 175 beats per minute. Because blood isn’t moving through the valves correctly, it can collect and cause clots, which can travel to the brain, blocking the flow of blood and causing a stroke.
AFib usually affects people 30 and 60 years old and is caused by heart disease or a heart abnormality. It’s also associated with obesity, sleep apnea, diabetes, hypertension and underlying heart issues.
Sometimes, AFib is discovered during routine physical exams when a doctor detects an abnormal heartbeat.
Other people notice symptoms, including the following:
- Chest pain
- Dizziness
- Racing heartbeat
- Shortness of breath
- Skipped heartbeat
- Weakness or fatigue
AFib can be treated very effectively, but early detection is key. If you experience any AFib symptoms, make an appointment with your doctor. They’ll review your family and personal medical history and perform a physical and an ECG to determine if AFib is the cause.

Advanced AFib Care You Can Trust
Our AdventHealth team of heart experts combines innovative treatment options with advanced expertise to offer the comprehensive, compassionate care you need to keep your heart beating properly.
Learn More About How the WATCHMAN FLX™ Works
Patient Testimonial - Gregory Kingery
Gregory Kingery of Sebring, Florida, traded warfarin for a WATCHMAN implant to treat his AFib. Learn how the change improved his quality of life.
What is Atrial Fibrillation?
Cardiac electrophysiologist James Irwin, MD, describes atrial fibrillation (AFib), its causes, symptoms and treatment options.
AdventHealth Tampa Medical Minute - WATCHMAN FLX - Dr. Paul Gerczuk
Paul Gerczuk, MD, outlines the advantages of the WATCHMAN and WATCHMAN FLX procedures and explains how the devices reduce the risk of stroke for people with AFib not caused by a heart valve issue.
AdventHealth Medical Moment: Watchman Procedure
Cardiac electrophysiologist Kenneth Yamamura, MD, and interventional cardiologist Asad Sawar, MD, discuss the WATCHMAN and WATCHMAN FLX procedures and detail how the device reduces stroke risk without using long-term blood thinners like warfarin.
More About the WATCHMAN FLX™
DISCLAIMER: The WATCHMAN™ and WATCHMAN FLX™ devices are permanent implants designed to close the left atrial appendage in the heart in an effort to reduce the risk of stroke.
With all medical procedures there are risks associated with the implant procedure and the use of the device. The risks include but are not limited to accidental heart puncture, air embolism, allergic reaction, anemia, anesthesia risks, arrhythmias, AV (Arteriovenous) fistula, bleeding or throat pain from the TEE (Trans Esophageal Echo) probe, blood clot or air bubbles in the lungs or other organs, bruising at the catheter insertion site, clot formation on the device, cranial bleed, excessive bleeding, gastrointestinal bleeding, groin puncture bleed, hypotension, infection/pneumonia, pneumothorax, pulmonary edema, pulmonary vein obstruction, renal failure, stroke, thrombosis, transient ischemic attack and, in some rare cases, death.
Be sure to talk with your doctor so you thoroughly understand all of the risks and benefits associated with the implantation of the device.
